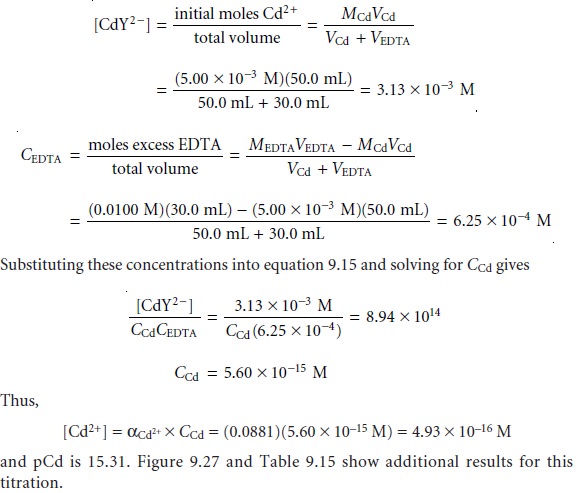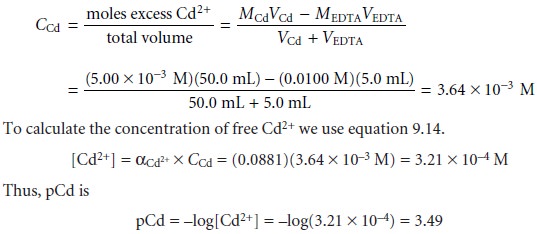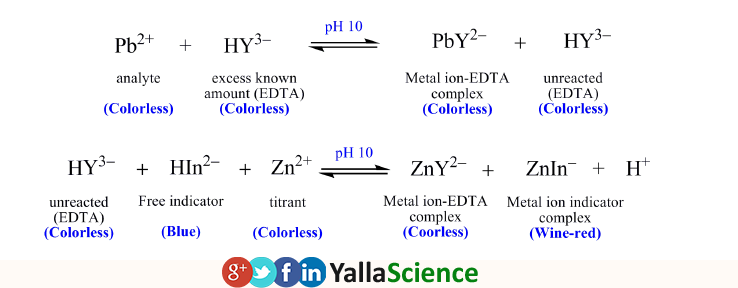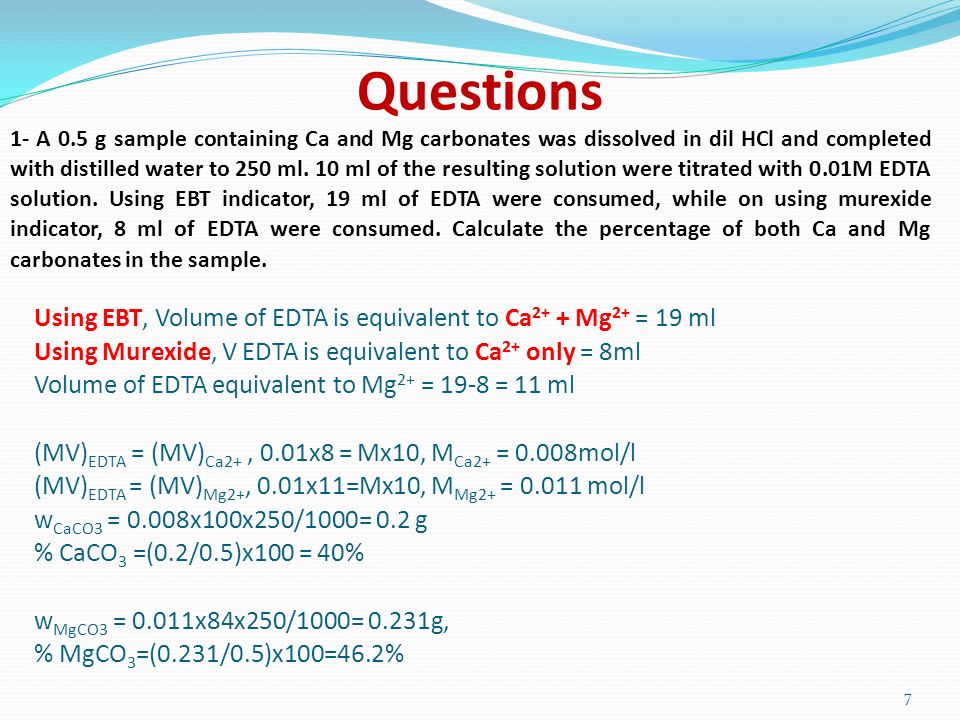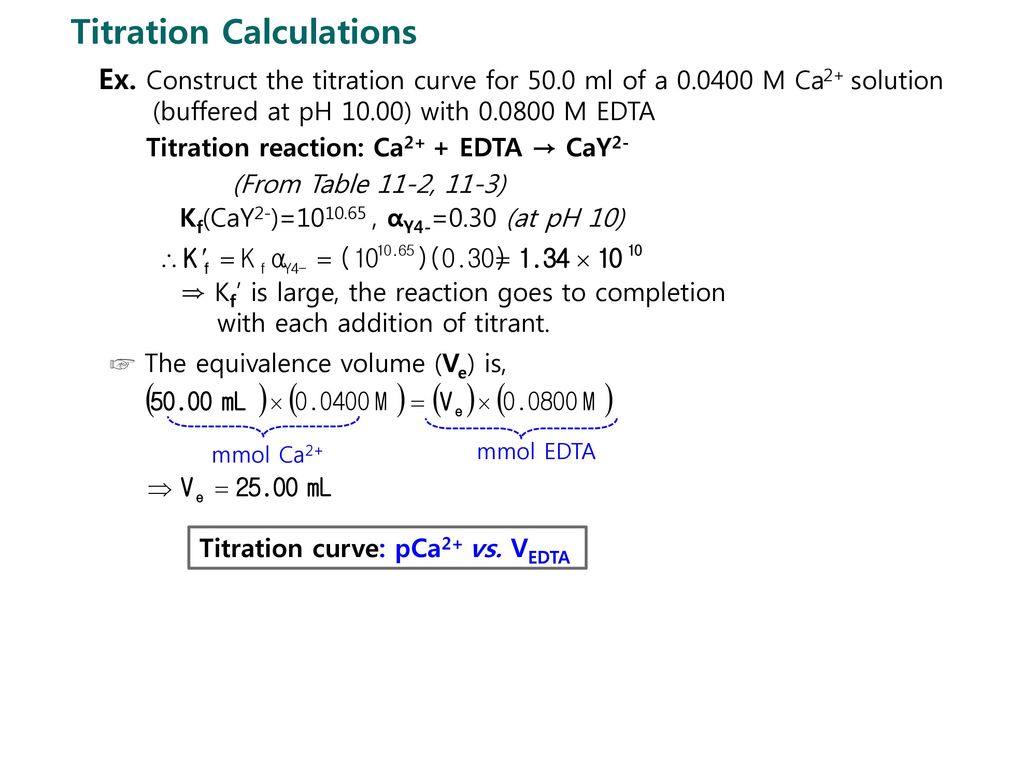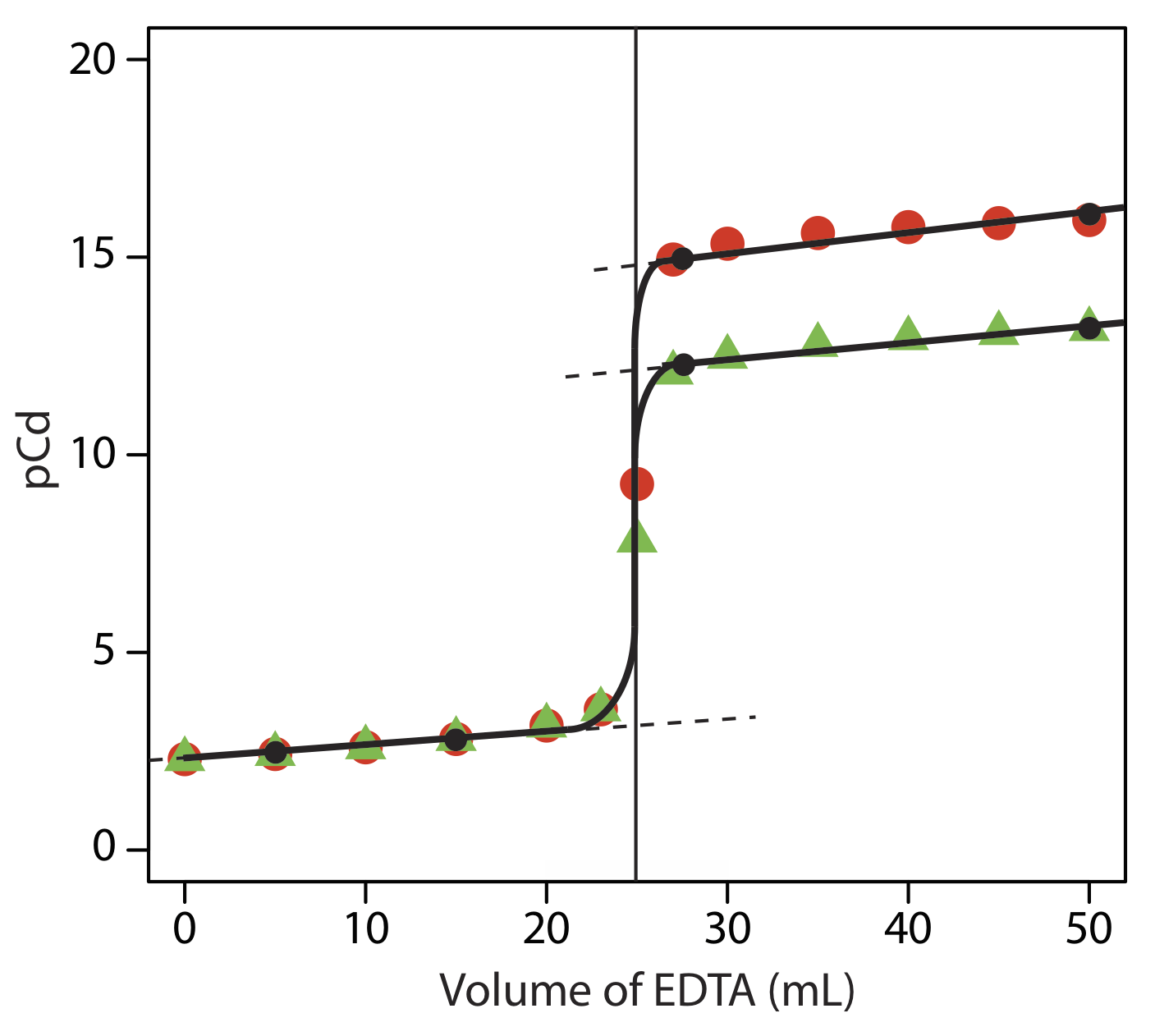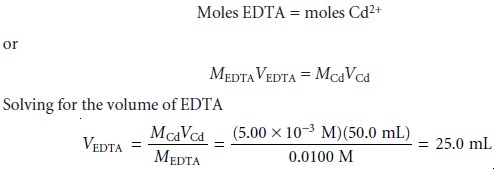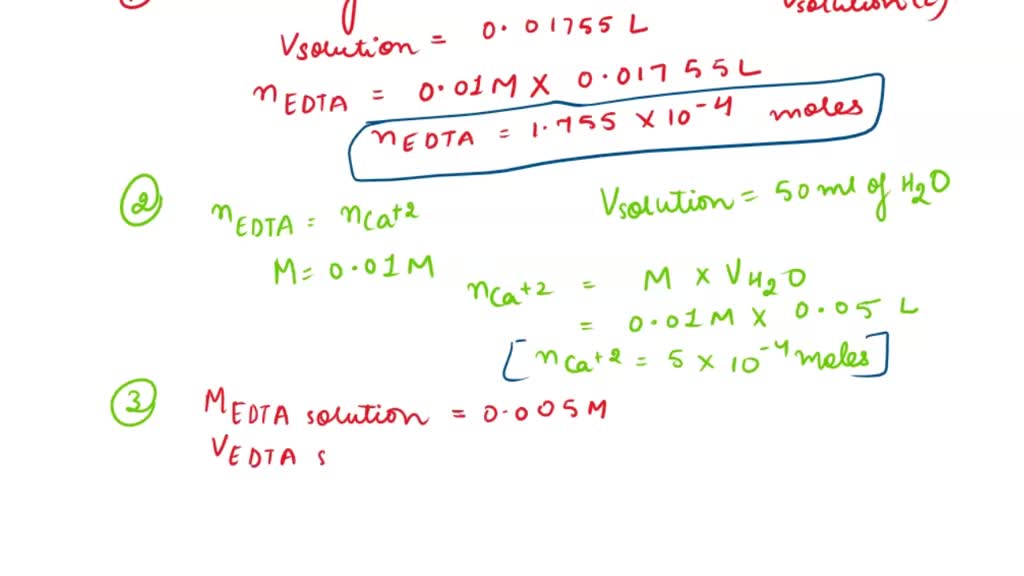
SOLVED: 1) Use the molarity of the EDTA solution and the average volumeof EDTA added to calculate the average number of moles of EDTArequired for the titration. Molarity EDTA= .01 Volume EDTA= .
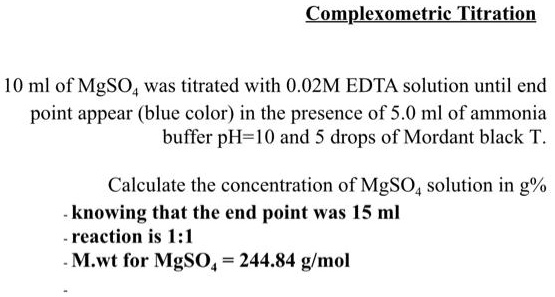
SOLVED: Complexometric Titration 10 ml of MgSO4 was titrated with 0.02M EDTA solution until end point appear (blue color) in the presence of 5.0 ml of ammonia buffer pH-I0 and 5 drops
SOLVED: 3 Back Titration: Ni?+ was determined in a complexometric titration using EDTA at pH 5.5 and xylenol orange as the indicator: The unknown solution containing 25.00 mL of Ni2+ was first