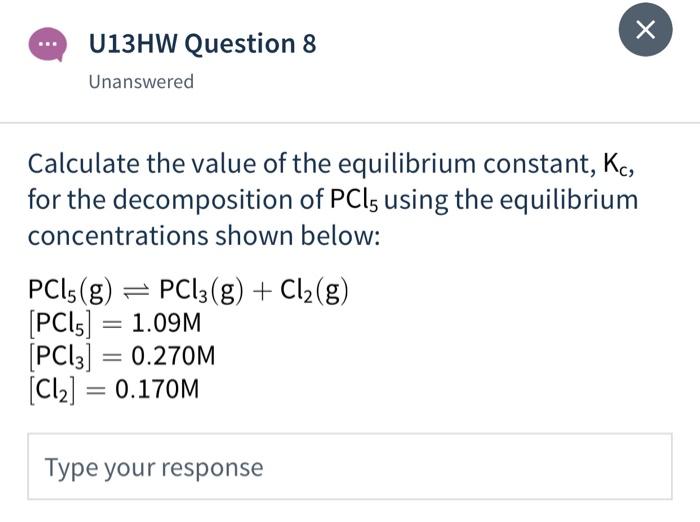
![SOLVED: 1o. Calculate PCls for the reaction below assuming that the equilibrium concentrations and equilibrium constant are as follows: Ko = 0.0042, [PCls] = 0.10M, [CI] = 0.10M PC15g=PC13g+Cl2g 11. Calculate [HCl] SOLVED: 1o. Calculate PCls for the reaction below assuming that the equilibrium concentrations and equilibrium constant are as follows: Ko = 0.0042, [PCls] = 0.10M, [CI] = 0.10M PC15g=PC13g+Cl2g 11. Calculate [HCl]](https://cdn.numerade.com/ask_images/04fdb71cae024bca934df0c5d38a25f1.jpg)
SOLVED: 1o. Calculate PCls for the reaction below assuming that the equilibrium concentrations and equilibrium constant are as follows: Ko = 0.0042, [PCls] = 0.10M, [CI] = 0.10M PC15g=PC13g+Cl2g 11. Calculate [HCl]
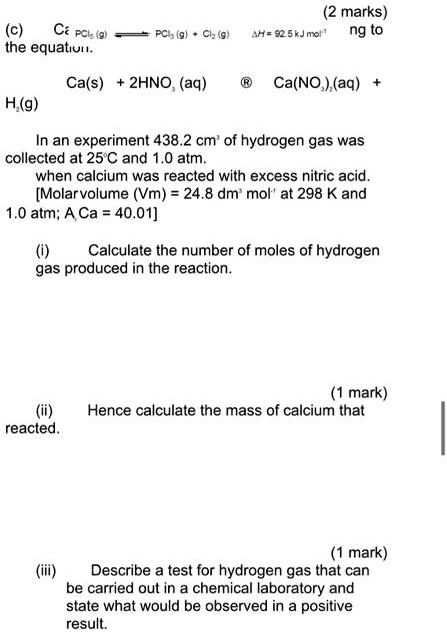
SOLVED: (2 marks) AA= 92 5kJnxi" ng to Ca PCls the equatiun PCIj (9 Ciz (9 Ca(s) 2HNO (aq) Ca(NO ) (aq) H(g) In an experiment 438.2 cm' of hydrogen gas was
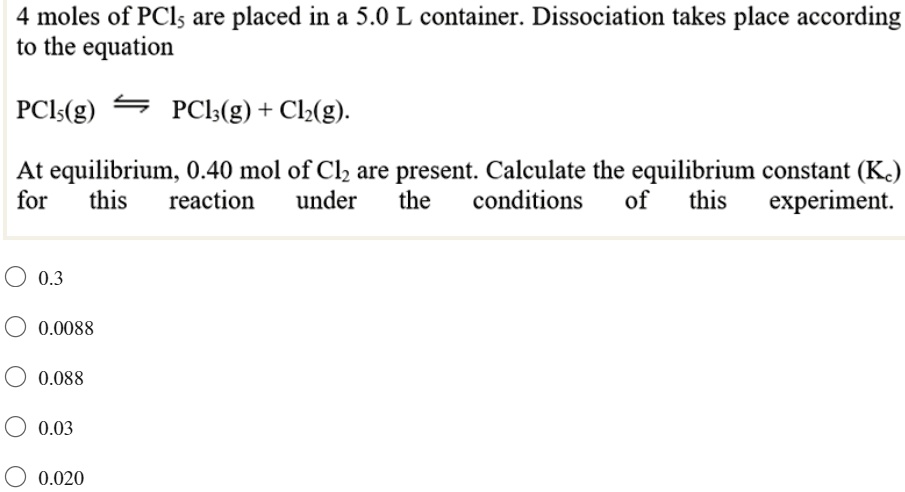
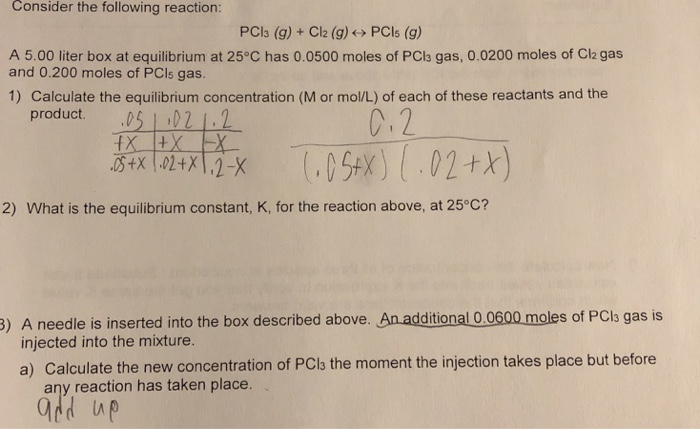
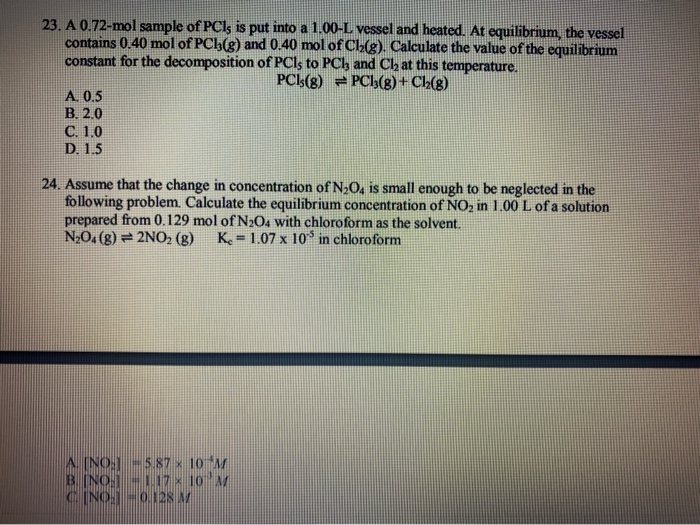
SOLVED: 4 moles of PCls are placed in a 5.0 L container: Dissociation takes place according to the equation PCls(g) PCls(g) + Ch(g). At equilibrium; 0.40 mol of Clz are present: Calculate

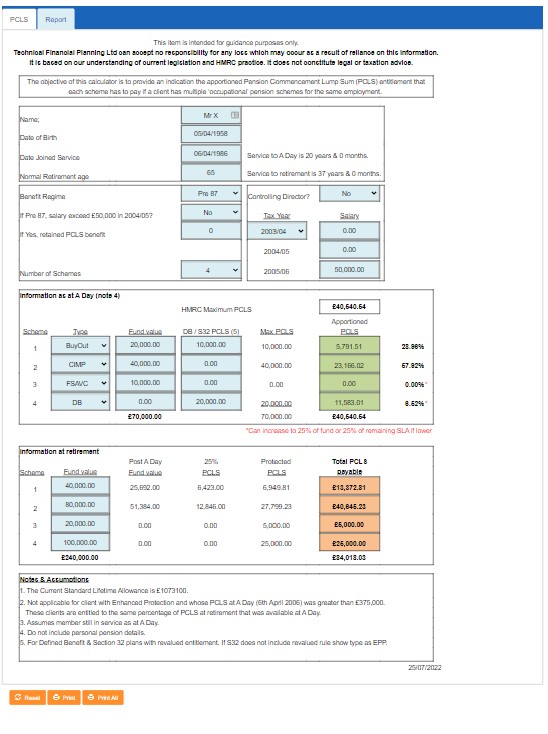
PCLS Meaning: What is a Pension Commencement Lump Sum || Maximum Tax-Free Pension Lump Sum - YouTube
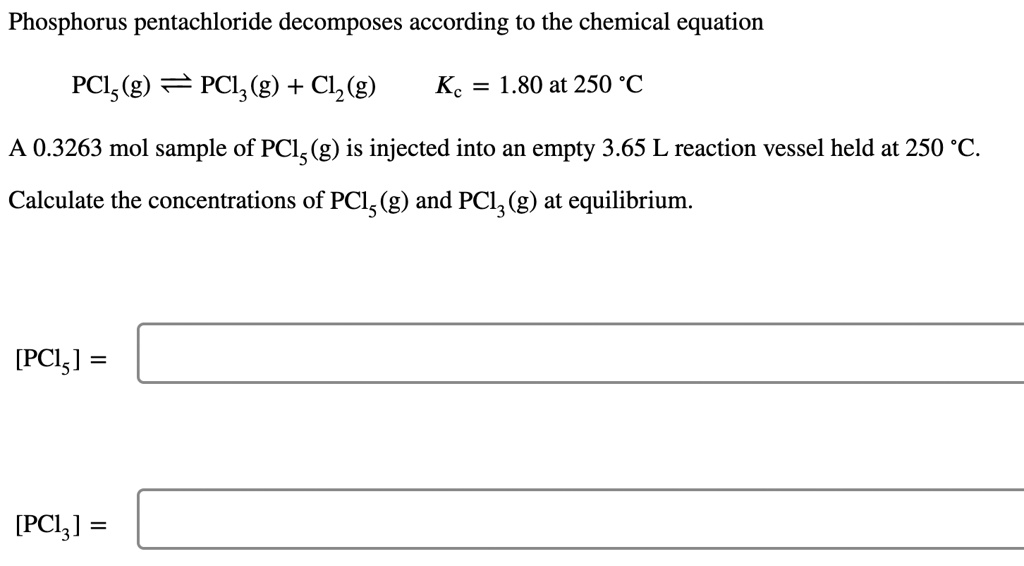
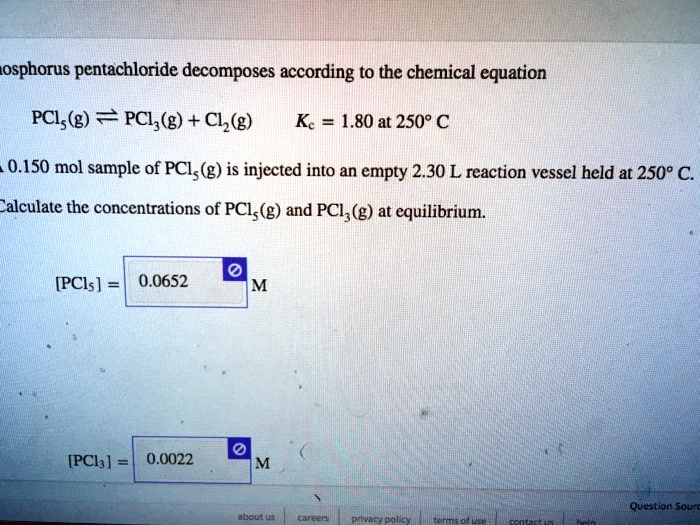
SOLVED: Phosphorus pentachloride decomposes according to the chemical equation PCls(g) = PCl3(g)+ C12(g) Kc= 1.80 at 250 C A 0.3263 mol sample of PCl (g) is injected into an empty 3.65 L
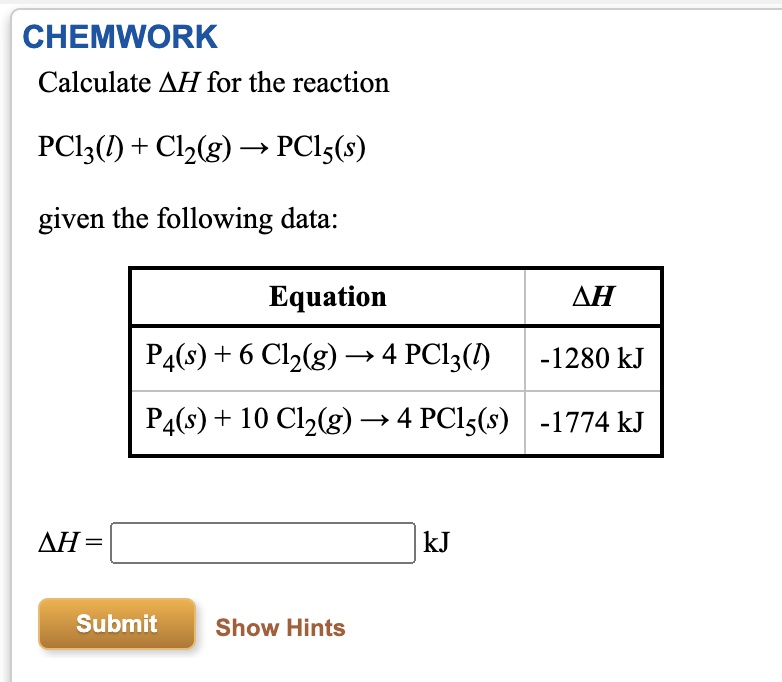
SOLVED: CHEMWORK Calculate AH for the reaction PClz(D) + Clz(g) = PCls(s) given the following data: Equation AH P4(s) + 6 Clz(g) 4 PClz() -1280 kJ Pa(s) + 10 Clz(g) - 4
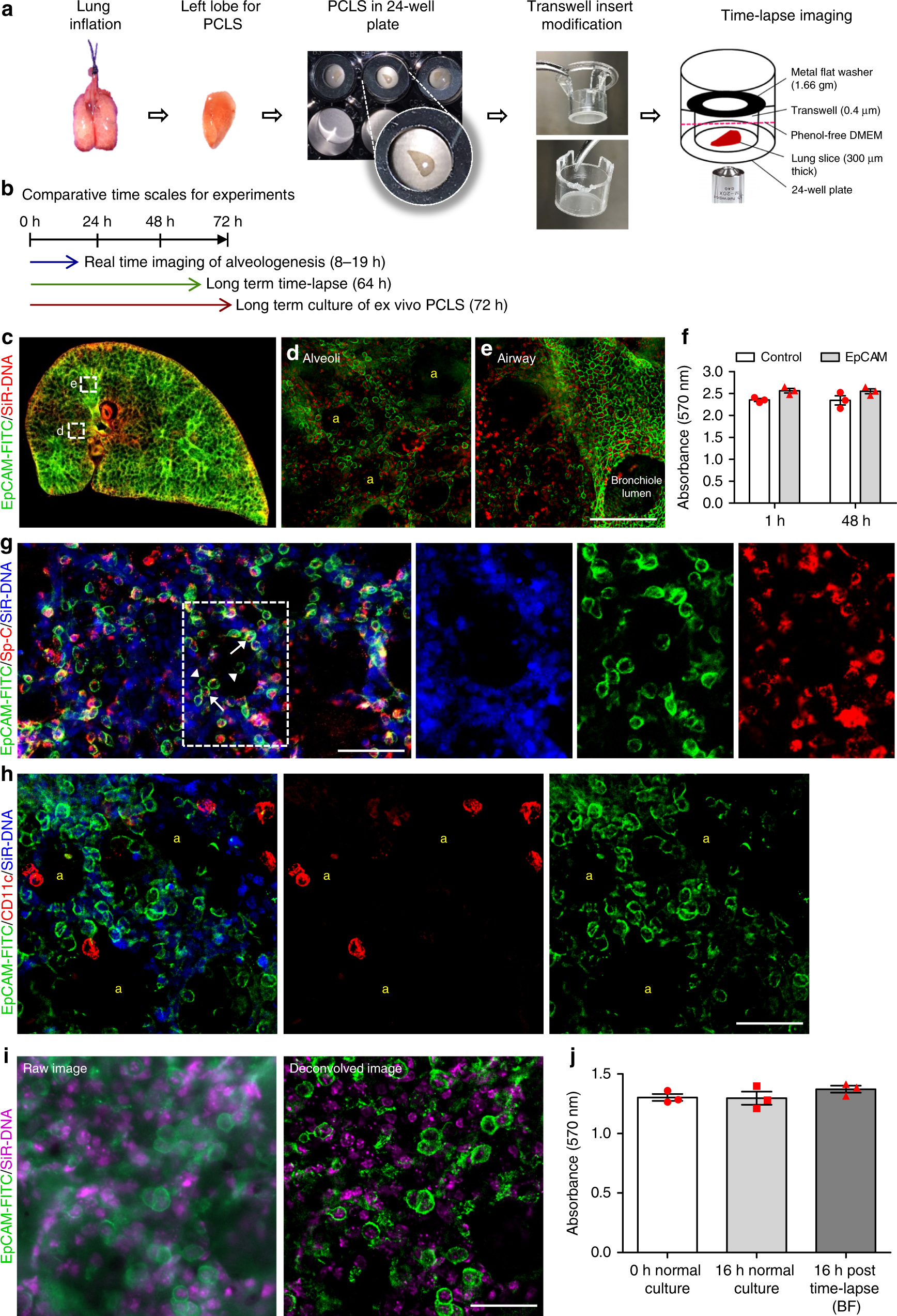
Live imaging of alveologenesis in precision-cut lung slices reveals dynamic epithelial cell behaviour | Nature Communications