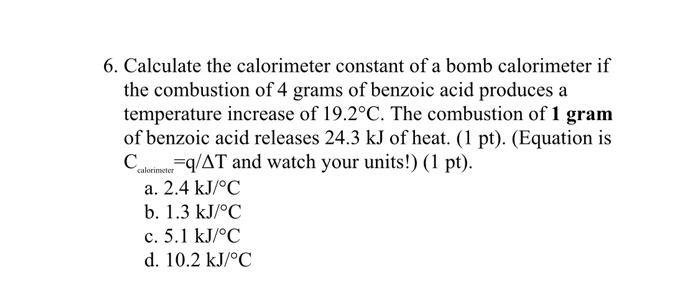
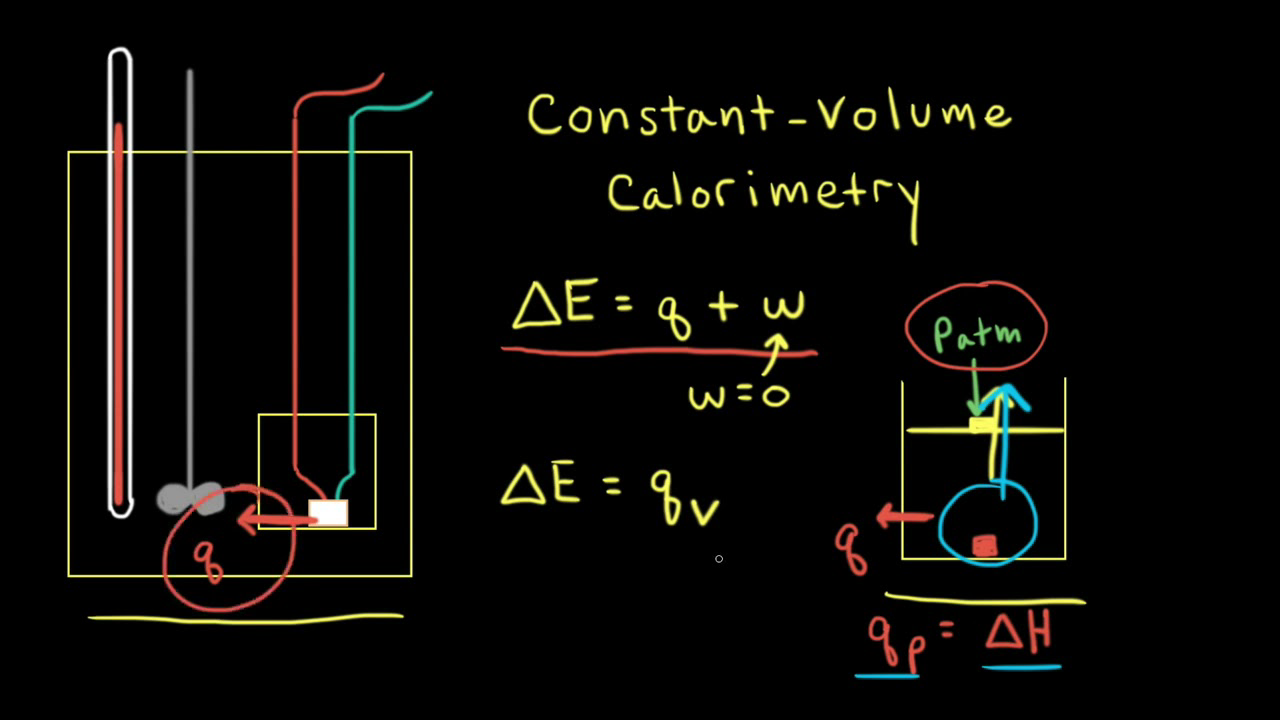
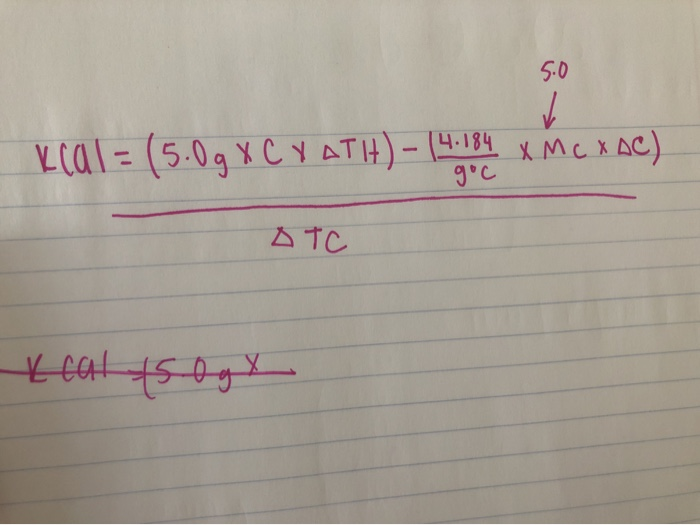How to Calculate the Heat of a Reaction from Constant-Pressure Calorimetry Data | Chemistry | Study.com

Calorimetry Calorimetry is used to measure heat capacity and specific heats. calorimeter: an instrument that measures heat changes for physical and chemical. - ppt video online download

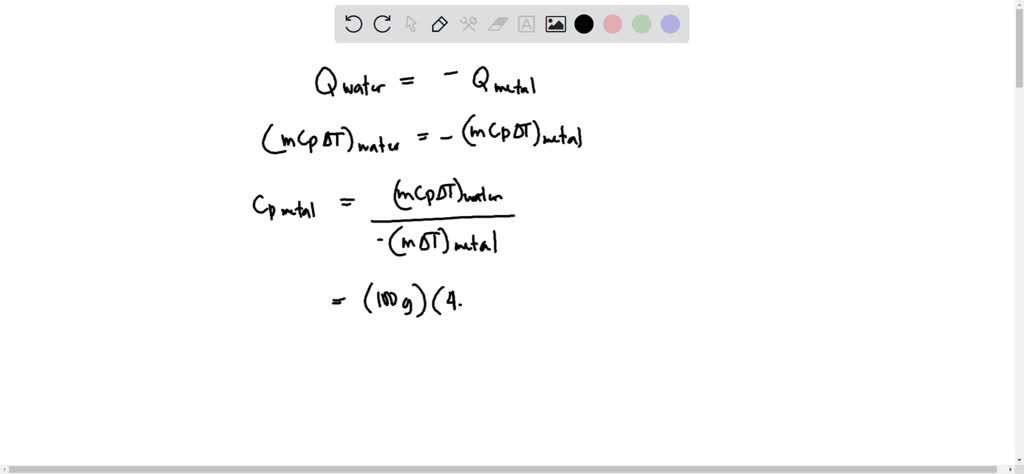
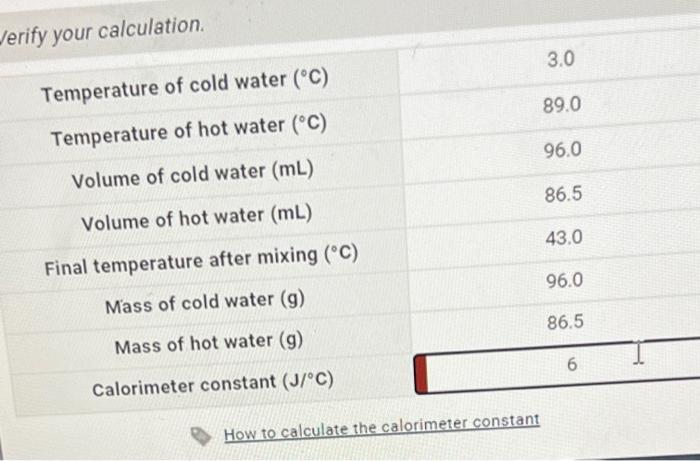
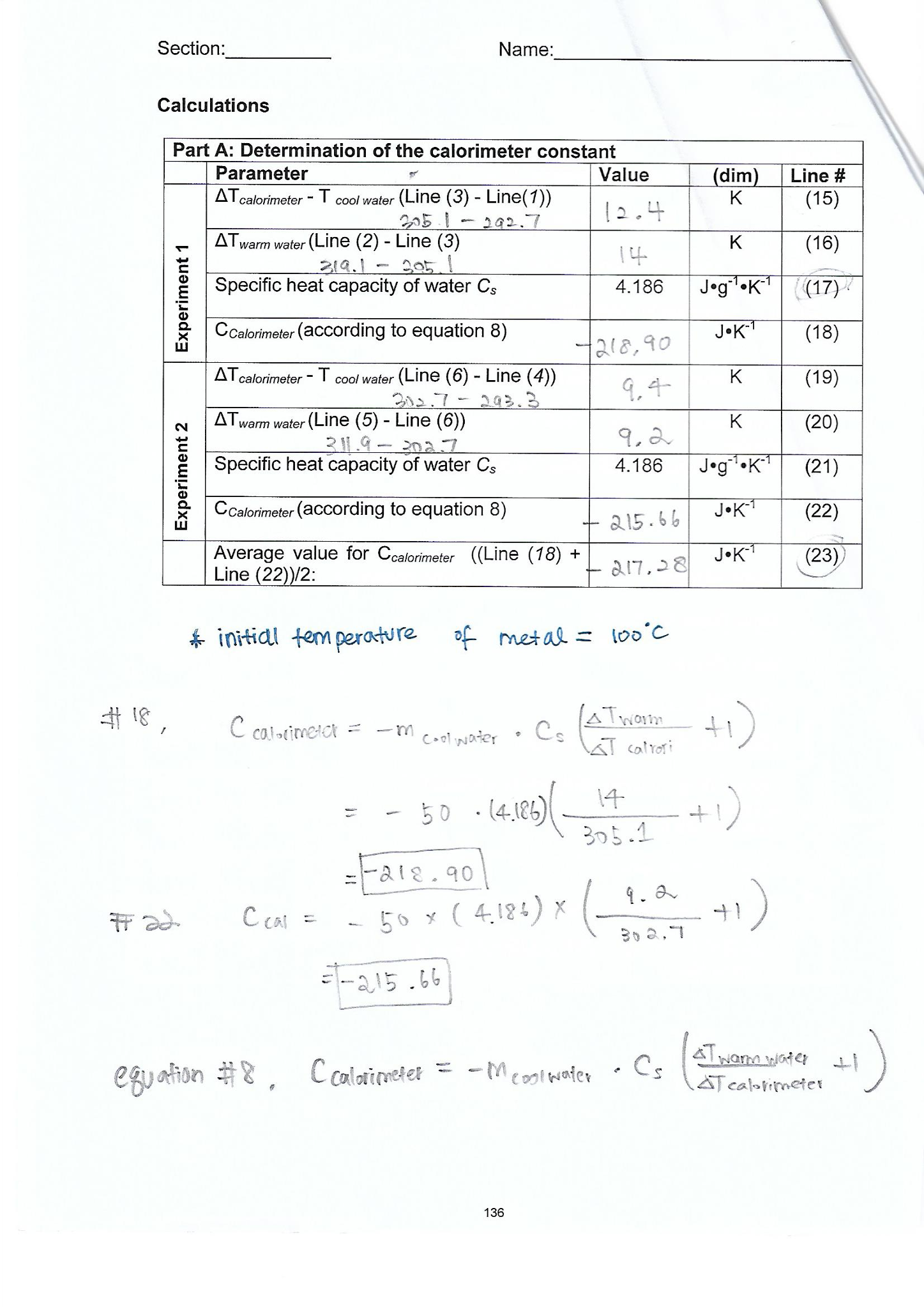
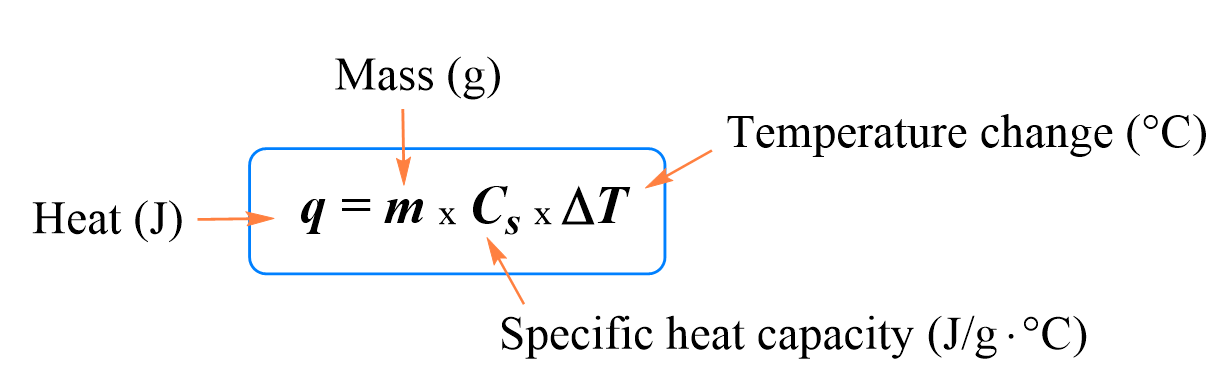
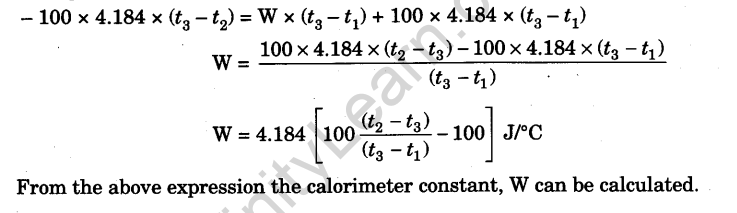
OneClass: In determining the calorimeter constant, a student added 50.00 mL H2O at 48.5oC to 50.00 mL...
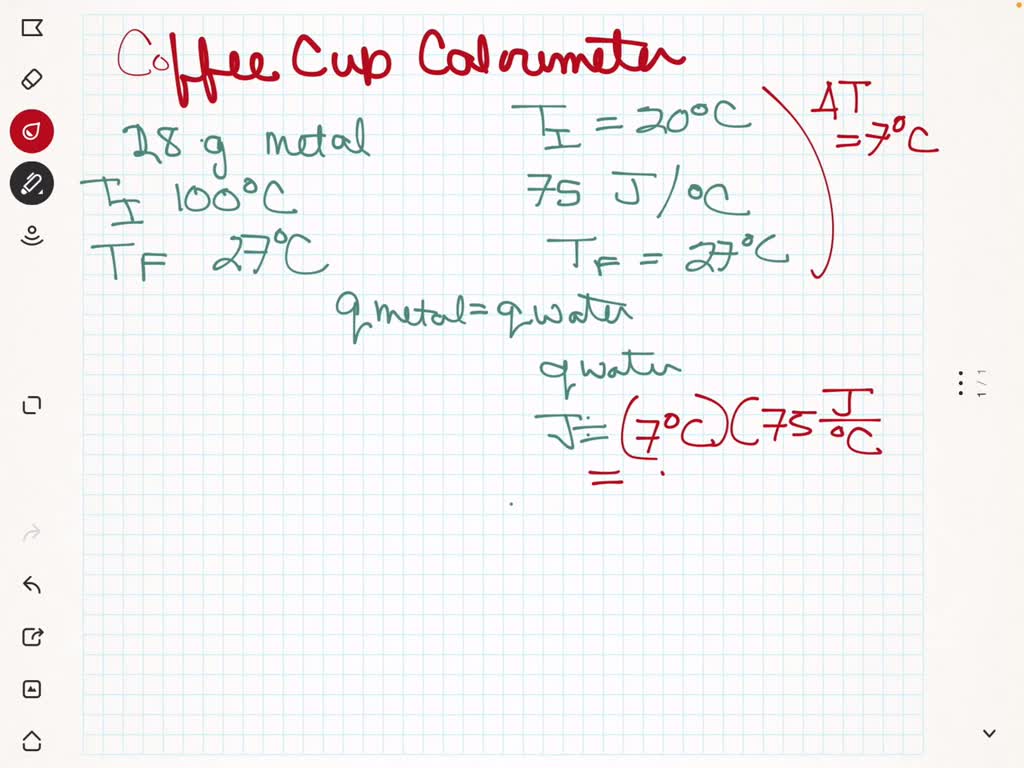
SOLVED: A coffee cup calorimeter contains water at an initial temperature of 20°C and is calculated to have a calorimeter constant (heat capacity) of 75 J/°C. A 28 g piece of an

Calorimetry Problems Chapter 6 part 3. Calorimetry Constant Pressure: measures enthalpy of rxn –coffee cup calorimetry Constant Volume: measures internal. - ppt download
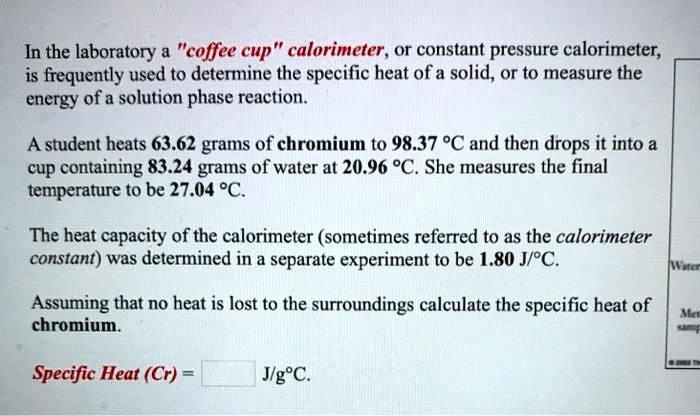
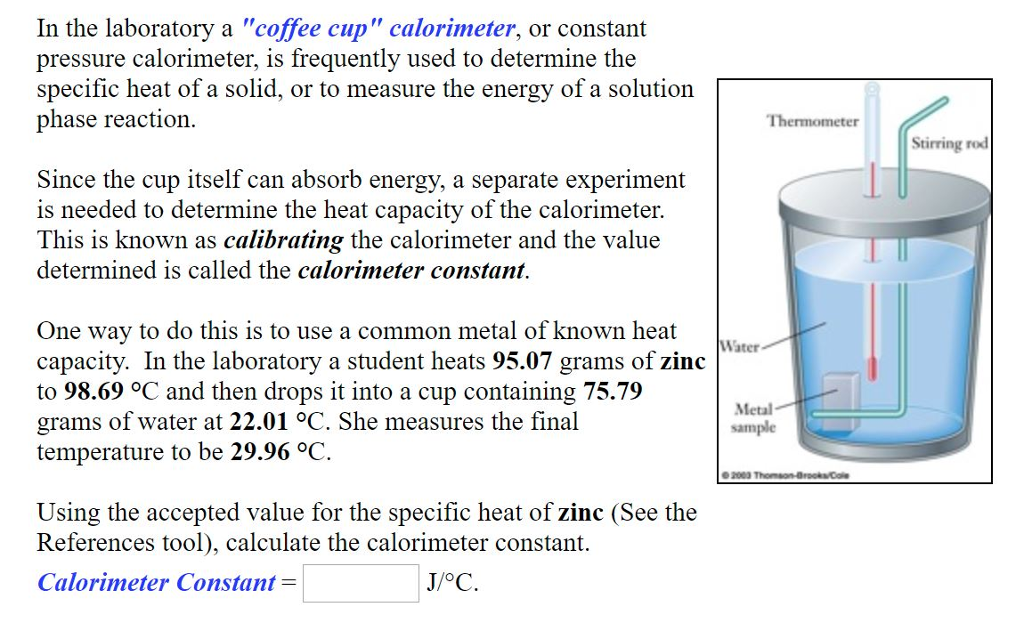
SOLVED: In the laboratory a "coffee cup" calorimeter , or constant pressure calorimeter; is frequently used to determine the specific heat of a solid, or to measure the energy ofa solution phase
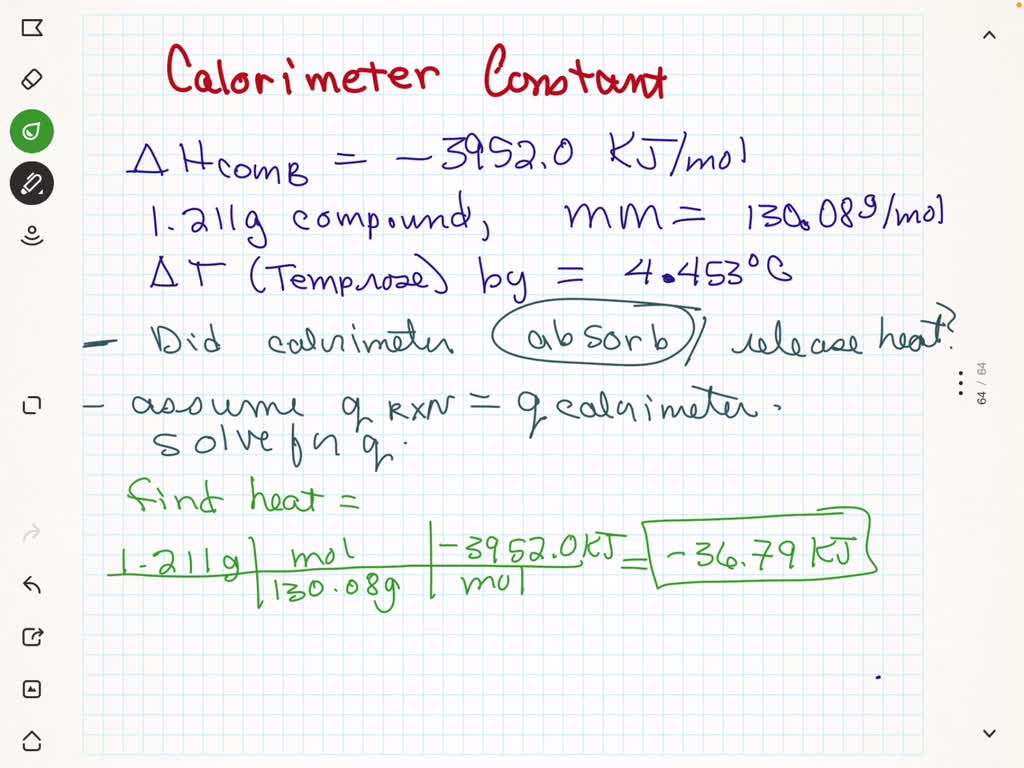
SOLVED: At constant volume, the heat of combustion of a particular compound is −3833.0 kJ/mol.−3833.0 kJ/mol. When 1.763 g1.763 g of this compound (molar mass=111.48 g/mol)(molar mass=111.48 g/mol) was burned in a