I have H2O2 of molecular wt 34.01gm and 30% w/v. What does it mean that I am not getting it and I want to prepare 0.1M solution, how can i? | ResearchGate
SOLVED: Calculate the final molarity of H2O2 if 5.2 mL of a 3.0% w/w H2O2 solution, which has a density of 1.0 g/mL, is added to 5.2 mL of a starch-iodide solution.
Welcome to Chem Zipper.com......: Calculate the Molarity of H2O2 if 11.2 ml H2O2 require 30 ml of 0.5 M K2Cr2O7 for its Oxidation . also calculate the volume of strength of H2O2.

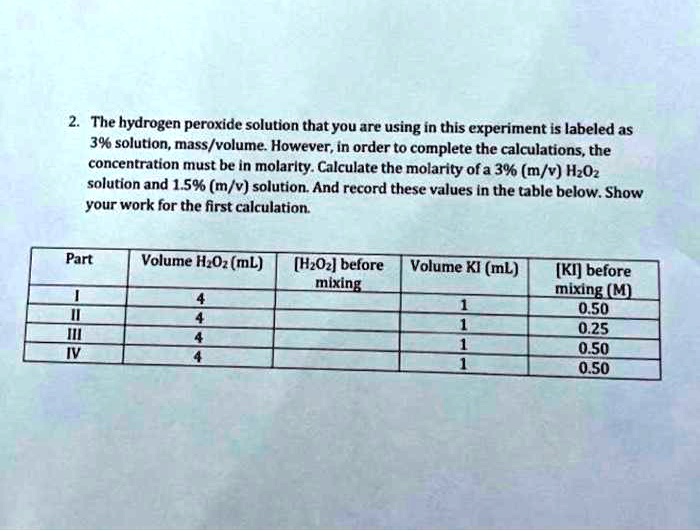
SOLVED: A solution of hydrogen peroxide is 30.0% H2O2 by mass and has a density of 1.11 g/cm3. Calculate the molarity of the solution. Show your calculation or expalin your answer.
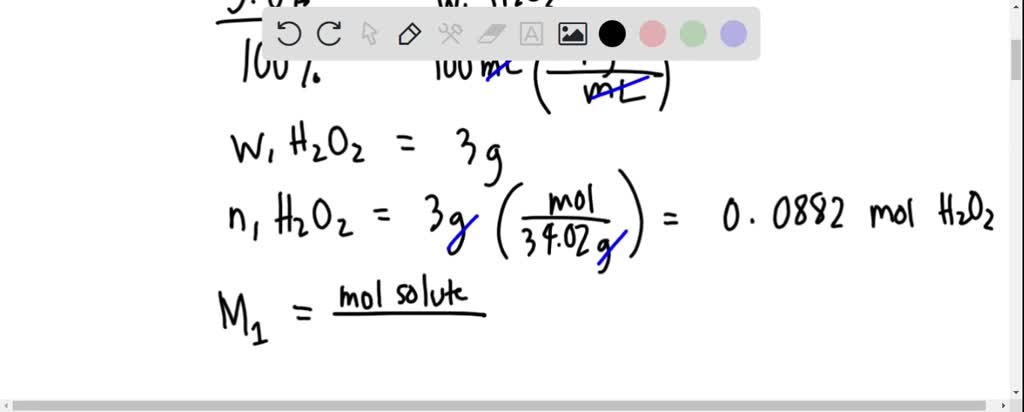
SOLVED: The hydrogen peroxide solution that you are using in this experiment is labeled as 3% solution; mass/volume However, in order to complete the calculations, the concentration must be in molarlty Calculate

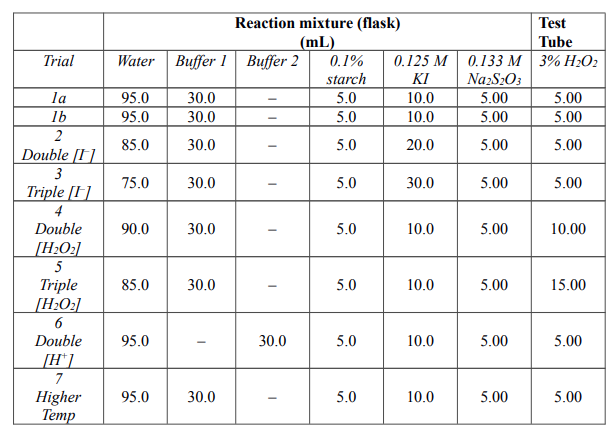
SOLVED: one method of determining the concentration of hydrogen peroxide ( H2O2) in a solution is through titration with the iodide ion. The net ionic equation is H2O2 + 2I-+2H+ –> I2+ 2H2O.
.gif)

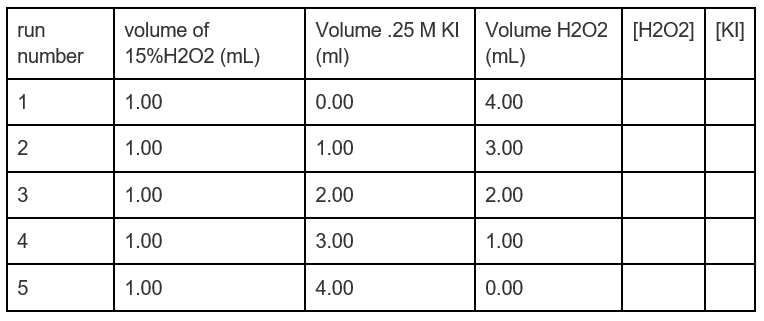

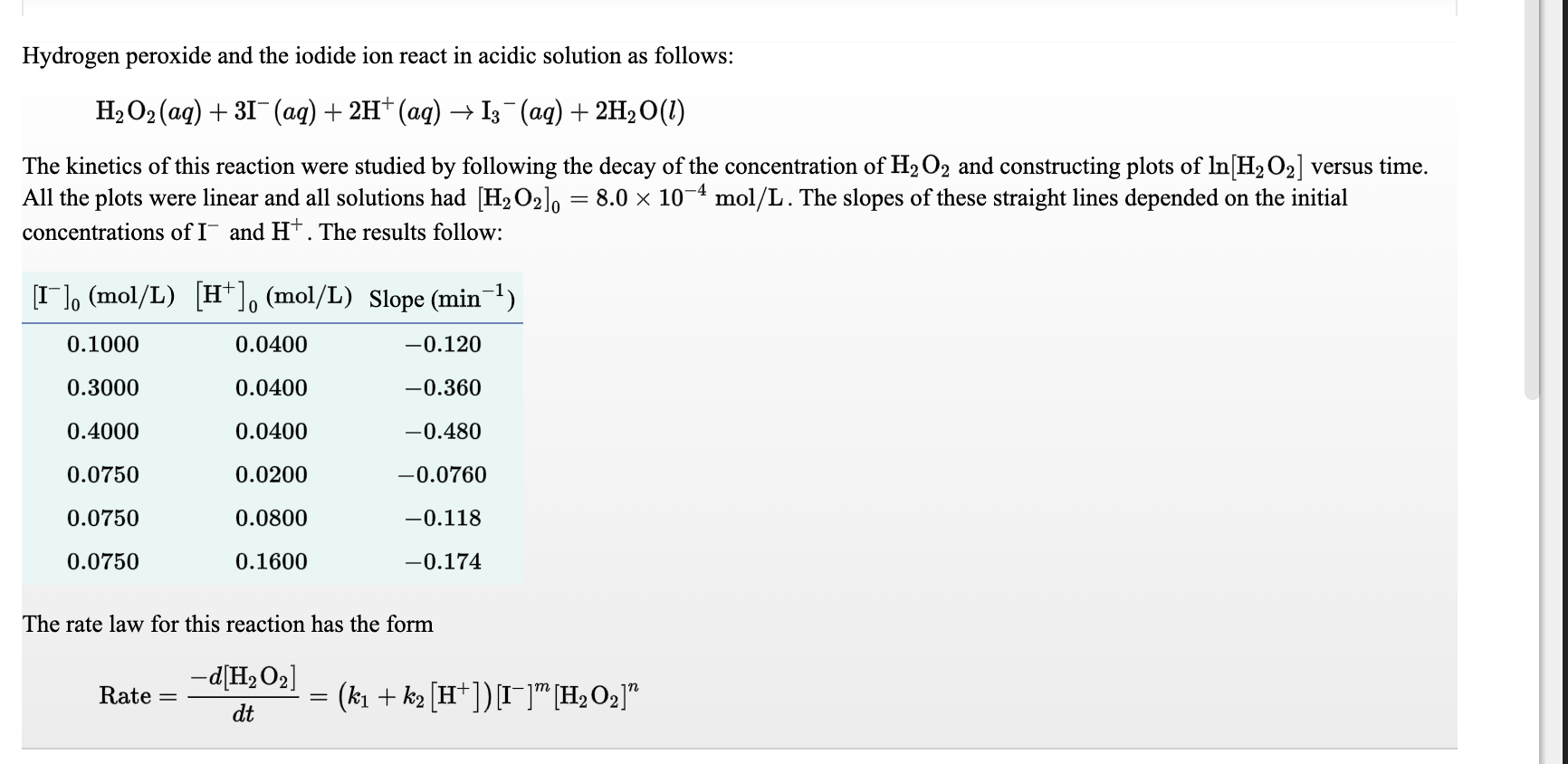




![The volume strength of 1 M H2O2 is:[Molar mass of H2O2 = 34 g mol^-1 ] The volume strength of 1 M H2O2 is:[Molar mass of H2O2 = 34 g mol^-1 ]](https://dwes9vv9u0550.cloudfront.net/images/2154655/640a9e6a-45be-4444-9242-719c87344f1c.jpg)