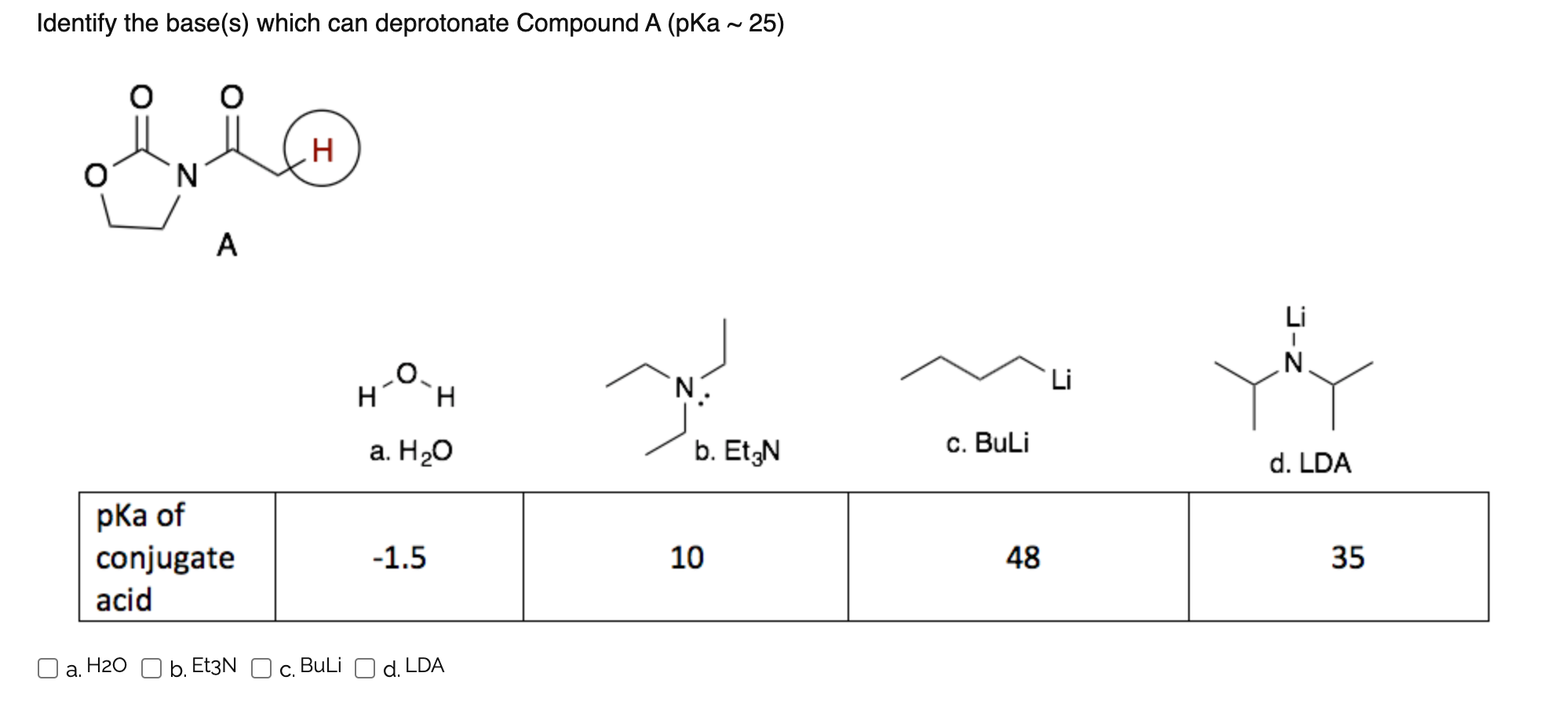
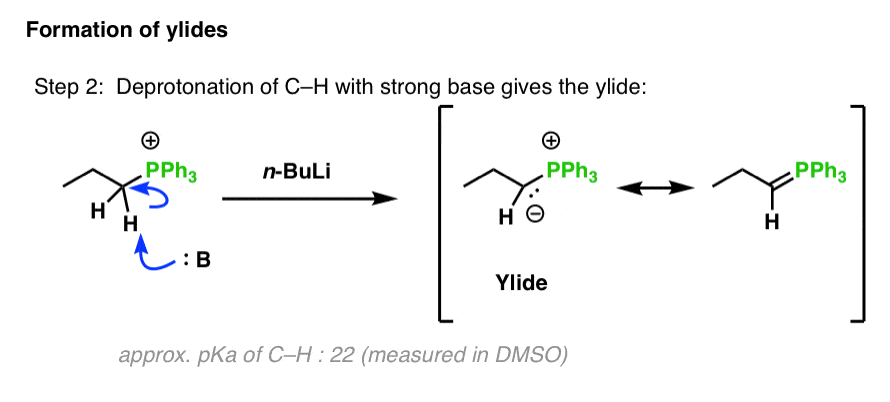
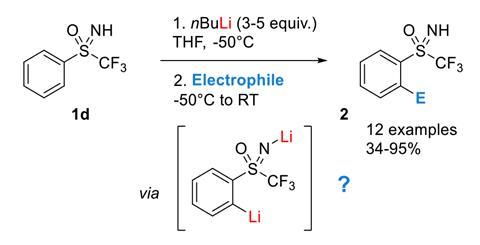
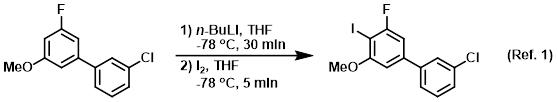NMR and DFT Studies with a Doubly Labelled 15N/6Li S‐Trifluoromethyl Sulfoximine Reveal Why a Directed ortho‐Lithiation Requires an Excess of n‐ BuLi - Hédouin - Angewandte Chemie International Edition - Wiley Online Library

Effect of Solvent on the Lithium−Bromine Exchange of Aryl Bromides: Reactions of n-Butyllithium and tert-Butyllithium with 1-Bromo-4-tert-butylbenzene at 0 °C | The Journal of Organic Chemistry
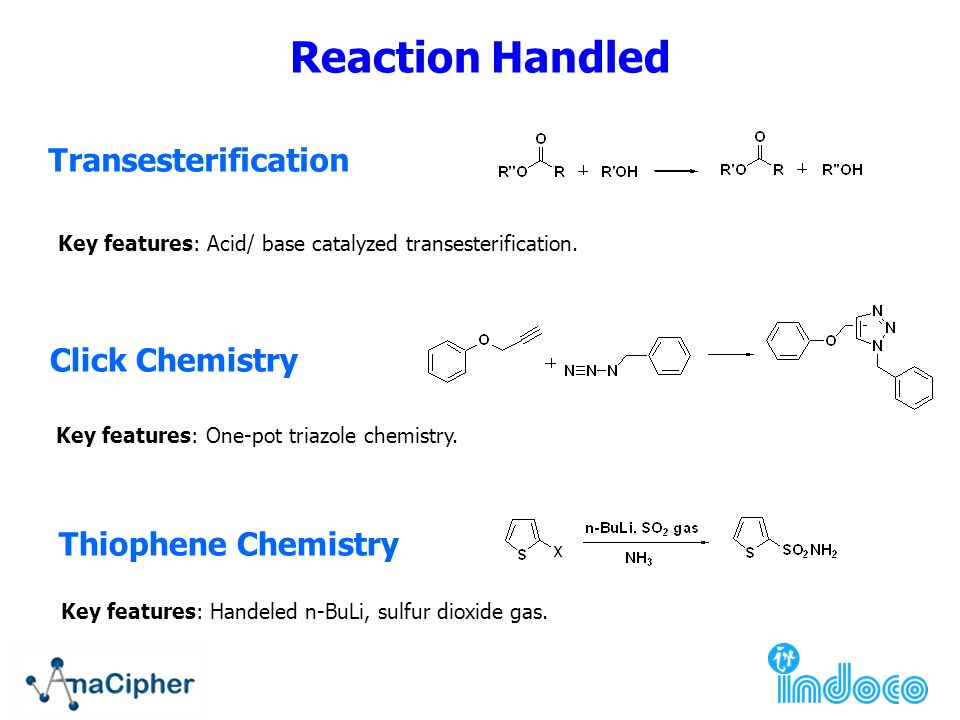
Grignard Reaction Key features: Handling of air/ moisture sensitive chemicals, formation of C-C bond. n-Butyl lithium Key features: Strong base such as. - ppt download
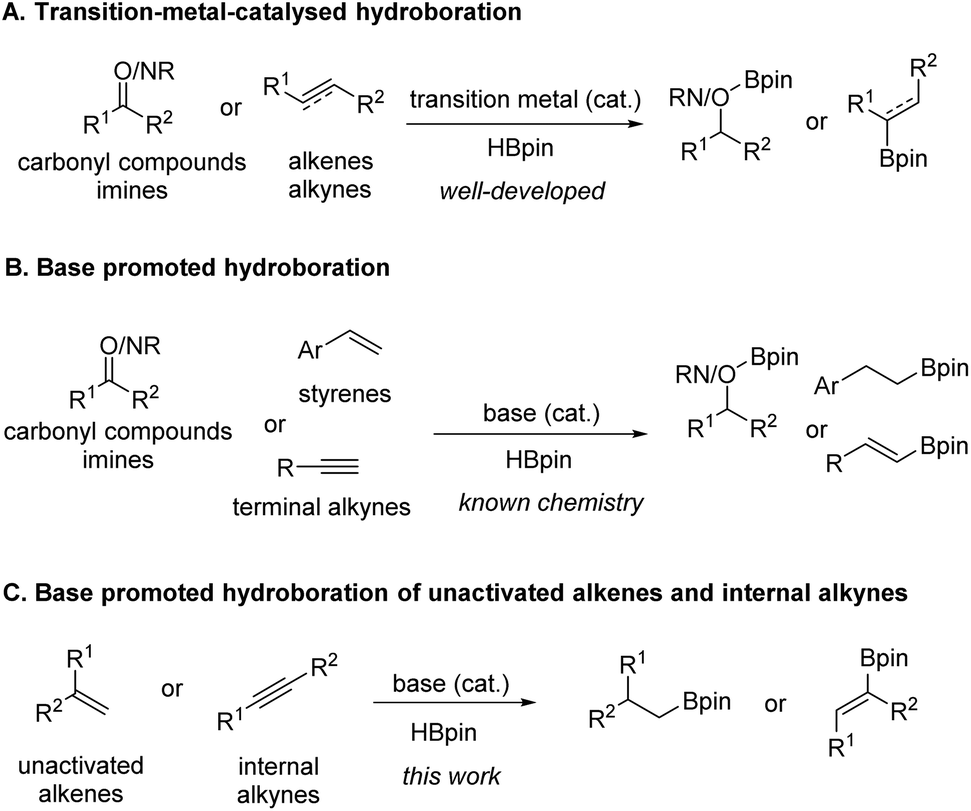
n BuLi-promoted anti -Markovnikov selective hydroboration of unactivated alkenes and internal alkynes - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C9QO00750D

Asymmetric Deprotonation using s-BuLi or i-PrLi and Chiral Diamines in THF: The Diamine Matters | Journal of the American Chemical Society

Mechanism of the Deprotonation Reaction of Alkyl Benzyl Ethers with n‐Butyllithium - Raposo - 2013 - Chemistry – A European Journal - Wiley Online Library